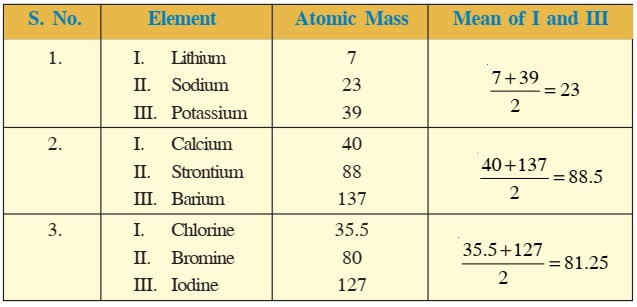
Dobereiner Triads were an early attempt at categorizing chemical elements in a periodic manner. They gave the earliest indication that elements can occur in families with similar properties. Their discovery set the stage for new advances in the periodic classification of elements.
Index
History
Dobereiner’s triads were first observed by Johann Wolfgang Dobereiner, a German chemist in the 19th century. He made observations about certain alkali earth metals and their salts. They formed a group of three, hence the name ‘triad’. He noticed that they had similar properties and their atomic masses followed a pattern. Later, by 1829, he could extend this theory to certain other ‘triads’ of elements.
All this suggested that some elements shared properties and could come under specific families.
Dobereiner Law of Triads
Dobereiner Law of Triads made the following observations:
- Some groups of three elements (‘triads’) shared common physical properties.
- The mean or average of the atomic weights of the heaviest and lightest elements was roughly or exactly equal to the atomic weight of the middle element.
- Some other physical properties (e.g.. density) also showed this trend. The average of the relevant property of the heaviest and lightest elements was very close to that of the middle element.
This lets scientists see similarities in different elements, and sort them based on similarities.
Applications of Dobereiner Triads
Dobereiner’s Triads were an early example of the periodic properties of elements. They inspired future scientists to make more attempts to categorize elements based on similar properties.
Such examples that followed include Newland’s Law of Octaves, and Lothar Meyer’s periodic classification. Eventually, this led to Mendeleev’s Periodic table and our Modern Periodic Table.
Examples of Dobereiner Triads
Here we present the original Dobereiner Triads examples.
Alkaline Earth Triad
This was the first triad to be discovered.
This consists of the elements calcium (\(\mathrm{Ca}\)), strontium (\(\mathrm{Sr}\)) and barium (\(\mathrm{Ba}\)).
Below their atomic masses are presented, in increasing order, where the pattern becomes clear.
| Element | Atomic Mass |
| Calcium | 40.1 |
| Strontium | 87.6 |
| Barium | 137.3 |
As we can see, the actual atomic weight of Strontium, the middle element, is \(87.6 \text{u}\). If we take the average of the first and last element’s atomic weights, we get, \(\frac{(40.1+137.3)}{2} = 88.7 \text{u}\) which is very close to \(87.6 \text{u}\).
Alkali Metal Triad
This consists consists of lithium (\(\mathrm{Li}\)), sodium (\(\mathrm{Na}\)) and Potassium (\(\mathrm{K}\)).
Tabulating their atomic masses, we see:
| Element | Atomic Mass |
| Lithium | 6.94 |
| Sodium | 23.0 |
| Potassium | 39.1 |
Clearly, the average of the atomic masses of lithium and potassium is \(\frac{(6.94+39.1)}{2} = 23.02 \text{u}\). This is almost exactly the atomic mass of sodium, \(23.02 \text{u}\).
Halogen Triad
The halogen elements also show these trends. They are also a Dobereiner triads example.
| Element | Atomic Mass |
| Chlorine | 35.4 |
| Bromine | 79.9 |
| Iodine | 126.9 |
Taking the average of the atomic masses of the heaviest and lightest elements, we have, \(\frac{(35.4+126.9)}{2} = 81.2 \text{u}\). This is reasonably close to the atomic weight of bromine, which is \(79.9 \text{u}\).
Chalcogen Triad
The chalcogen triad consists of elements Sulphur (\(\mathrm{S}\)), Selenium (\(\mathrm{Se}\)) and Tellurium (\(\mathrm{Te}\)) from the oxygen family.
| Element | Atomic Mass |
| Sulphur | 32.1 |
| Selenium | 78.9 |
| Tellurium | 127.1 |
Again, the average of the first and light element atomic masses gives us \(79.9 \text{u}\), very close to the atomic mass of \(\mathrm{Se} , 78.9 \text{u}\).
FAQs
Dobereiner Triads are sets of three chemical elements that share similar physical and chemical properties. It was an early attempt at classifying elements.
There were originally 4 sets of Dobereiner Triads in all.
They are listed here:
1. Calcium (\(\mathrm{Ca}\)), strontium (\(\mathrm{Sr}\)) and barium (\(\mathrm{Ba}\)).
2. Lithium (\(\mathrm{Li}\)), sodium (\(\mathrm{Na}\)) and Potassium (\(\mathrm{K}\)).
3. Chlorine (\(\mathrm{Cl}\)), bromine (\(\mathrm{Br}\)) and Iodine (\(\mathrm{I}\)).
4. Sulphur (\(\mathrm{S}\)), Selenium (\(\mathrm{Se}\)) and Tellurium (\(\mathrm{Te}\).
As the atomic mass of strontium, \(87.6 \text{u}\), is very close to the average \(\frac{(40.1+137.3)}{2} = 88.7 \text{u}\) of Calcium and Barium, Ca Sr Ba are a Dobereiner Triad.
No, Fe Co Ni are not a Dobereiner triad, as the atomic weight of the middle element is not the average of the first and the last.