The difference between Emission and Absorption Spectra is self-explanatory where Absorption Spectrum constitutes the missing incident frequencies of radiation which are absorbed by the sample material when its electrons transition from a low energy state to a high energy state. Whereas, Emission Spectrum constitutes the frequencies of radiation emitted when electrons of the material transition from a high energy state to a low energy one to attain stability.
Index
Absorption Spectrum
There are different types of absorption spectrum depending on the state of the molecule being changed. Some of these are:
- Rotational Lines, when the rotational state of a molecule changes.
- Vibrational Lines, when the vibrational state of a molecule changes.
These above lines can also occur in a combination, like Rotational + Vibrational Spectrum.
Depending on the frequency of radiation being absorbed, these lines lie in various regions of the spectrum of electromagnetic radiation. For example, rotational lines are mostly found in the microwave band of the spectrum.
There are many factors which can cause the frequency of an absorption line to shift. Some of these are:
- Electric and magnetic fields
- Neighbouring molecules
Emission Spectrum
Similar to the absorption spectrum, the emission spectrum also has different types of lines depending on the state of the molecule being changed by the incident radiation.
- Rotational Lines
- Vibrational Lines
- Vibronic Lines (Combination of Vibrational and Electronic Lines)
Difference Between Emission and Absorption Spectra
Now, since we know what these two spectra are. Let’s have a look at the difference between emission and absorption spectra.
| Absorption Spectrum | Emission Spectrum |
|---|---|
| Formed from the absorption of incident frequencies by the material. | Formed by radiation released by electrons transitioning from a high energy state to low energy state to attain stability. |
| Consists of black lines of absorbed frequencies in between the continuous spectrum of incident frequencies. | Consists of lines of frequencies of emitted radiation in an empty black band. |
| Can determine the different types of compounds present in a mixture. | Can determine the elements present in a given sample material. |
| The intensity of spectral lines gives the number of elements present in the mixture. | The intensity of spectral lines gives the amount of an element present. |
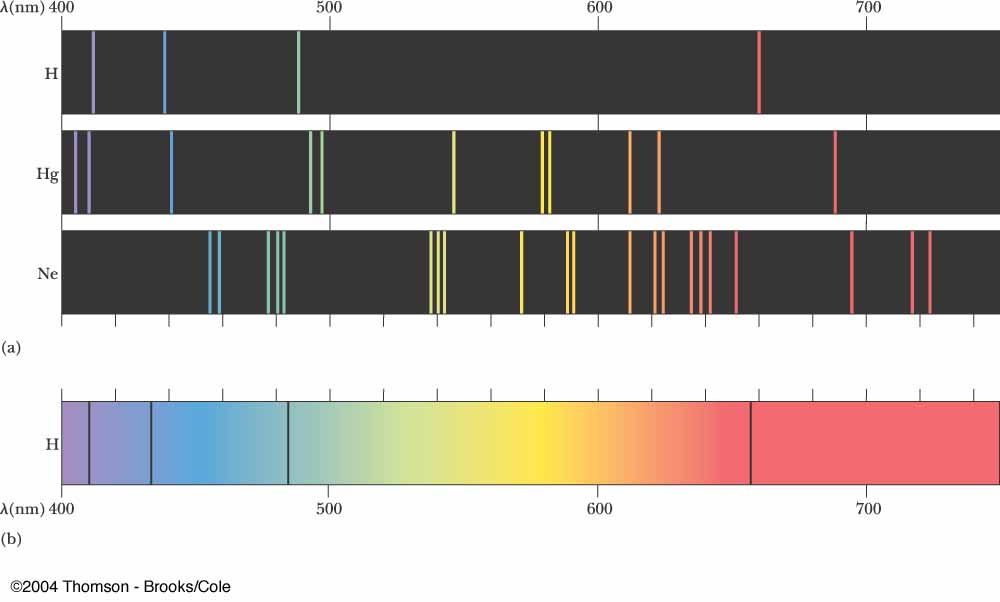
(Source)
FAQs
Absorption and emission spectra are related in the sense that, absorption can occur at any frequency that emission is occurring for the material, but not vice versa. Also, absorption spectrum can be obtained from an emission spectrum using Einstein’s coefficients.
Absorption spectra are used in many fields due to their unique property of distinguishing the constituents of a mixture. Some of these are:
1. Astronomy: To find the environmental conditions of an extrasolar planet and also their discovery. Also used to find the composition of interstellar clouds.
2. Chemical Analysis: Can be used to identify the pollutants in a region/portion of air.
3. Remote Sensing: Measurements and analysis of a sample can be done from a distance without getting into contact with it. For example, analysis of samples/objects present in toxic or dangerous environments.
4. Atomic and Molecular Physics: Can be used to determine the properties of an atom or molecule, like electronic structure and molecular mass. Can also be used to determine the accuracy of theories, like Lamb shift.
Many applications of emission spectra are because every element in the periodic table has a unique emission spectrum. Some of these are:
1. Astronomy: Used to find the composition of stars.
2. Research: Used to analyze and investigate the electronic and geometric structure of transition elements.
Spectroscopy is the a branch of science which is used to investigate and analyze electromagnetic radiation when it interacts with molecules or atoms.
