Amicetin is an antibacterial and antiviral agent belonging to a group of disaccharide nucleoside antibiotics featuring an \(\alpha-(1 \rightarrow 4)\)-glycosidic bond. Nucleoside antibiotic is a large family of secondary microbial metabolites that exhibit diverse and potent bioactive properties.
Some other examples of Nucleoside antibiotics are plicacetin, cystosaminomycin A-D. Amicetin is a disaccharide pyrimidine nucleoside antibiotic produced by Streptomyces fasciculatis and Streptomyces vinaceusdrappus.
Index
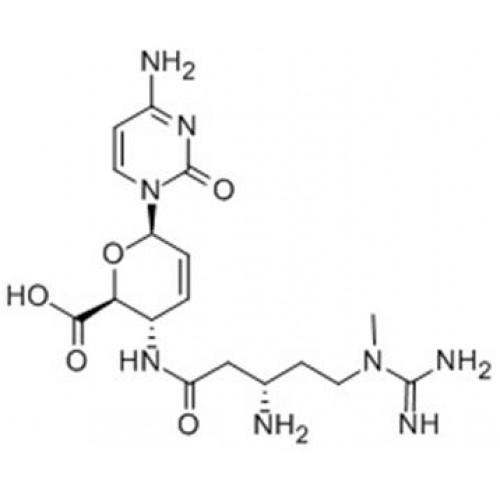
Structure & Formula
The IUPAC name of amicetin is:
4-[[(2S)-2-amino-3-hydroxy-2-methylpropanoyl]amino]-N-[1-[(2R,5S,6R)-5-[(2R,3R,4S,5S,6R)-5-(dimethylamino)-3,4-dihydroxy-6-methyloxan-2-yl]oxy-6-methyloxan-2-yl]-2-oxopyrimidin-4-yl]benzamide
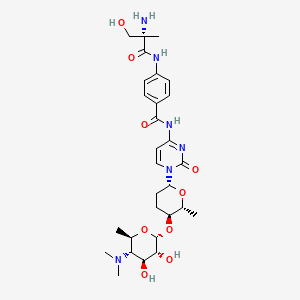
The molecular formula of Amicetin is C29H42N6O9.
It belongs to a group of disaccharide nucleoside antibiotics featuring an \(\alpha-(1 \rightarrow 4)\)-glycosidic bond.
The structure of Amicetin consists of a Nucleobase, Disaccharide and Amino Acid Moieties.
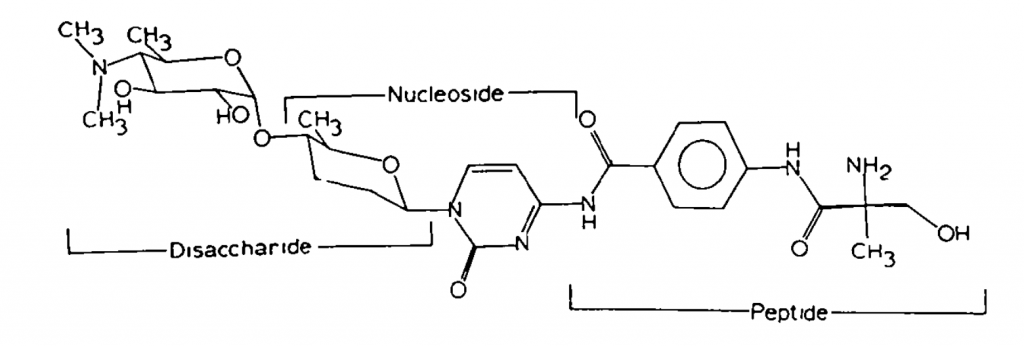
Origin of Amicetin
The drug was first identified by Boer Clarence De and Jack W Hinman in 1951. In 1953, amicetin was first isolated from Streptomyces fasciculatus and Streptomyces vinaceus drappus. Upjohn Co applied for a patent for Amicetin. The patent expired in 1976. Currently, there is no patent for it.
The chemical structure of this antiviral agent was established in 1962 after extensive studies of its hydrolytic products and was finally confirmed by crystallization studies.
The chemical has been known for its activity against a number of both Gram-negative and Gram-positive bacteria, particularly Mycobacterium tuberculosis, and was also found to have effects against herpesvirus 1 and poliovirus.
Pharmacological Effect
This antibacterial and antiviral agent has been described as a universal antibiotic, exerting effects against microorganisms of different evolutionary origins, including archaea, bacteria, and eukarya, by functioning as a peptidyl transferase inhibitor to block protein biosynthesis.
Mechanism of Action
Like a number of other structurally unrelated antibiotics, amicetin interfered with the resynthesis of M protein which had been removed with trypsin from a strain of Streptococcus pyogenes group A.
Since the same concentration of this inhibited both growth and protein synthesis, it was suggested that the antibiotic inhibits growth because it inhibits protein synthesis.
The addition of amicetin to logarithmically growing cultures of E. coli inhibits protein synthesis predominantly and affects the formation of RNA and DNA only a little. While protein synthesis ceases at once, production of both nucleic acids continues, although at a slower rate. It inhibits protein synthesis by blocking peptide synthesis following aminoacyl-RNA formation.
An aminoacyl-tRNA cannot bind until a binding complex is formed with the elongation factor EF-Tu and GTP (guanosine triphosphate). The binding of this complex leads to the ejection of the deacylated tRNA from the E site.
The new aminoacyl-tRNA binds to the A site and is aligned with the peptidyl-tRNA at the P site, and a peptide bond is formed by the action of peptidyltransferase. Peptidyltransferase activity is catalyzed by 23S ribosomal RNA (a ribozyme).
Amicetin affects the activity of 23S ribosomal RNA by changing its structure by binding to it. Although the binding of this drug to a conserved structural motif of 23S rRNAs has been investigated using site-directed mutations, chemical footprinting, nuclear magnetic resonance (NMR) structure, and molecular modeling studies, the exact working mechanism has been investigated for amicetin remains elusive.
Applications of Amicetin
- Amicetin is an antibacterial and antiviral agent.
- The drug is for its activity against a number of both Gram-negative and Gram-positive bacteria, particularly Mycobacterium tuberculosis.
- It is found to work against herpesvirus 1 and poliovirus also.
- It has exerting effects against microorganisms of different evolutionary origins, including archaea, bacteria, and eukarya.
- By functioning as a peptidyl transferase inhibitor, it helps block protein biosynthesis.
Similar Drugs
Plicacetin
Cytosaminomycin A—D
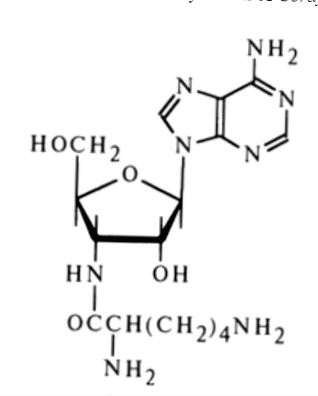
Lysylaminoadenosine
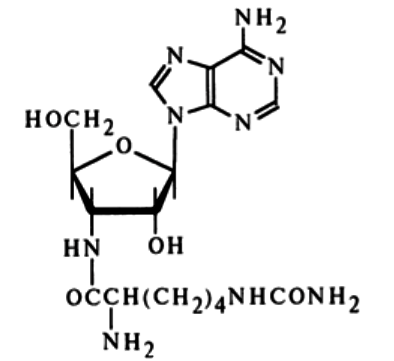
Homocitrullylaminoadenosine
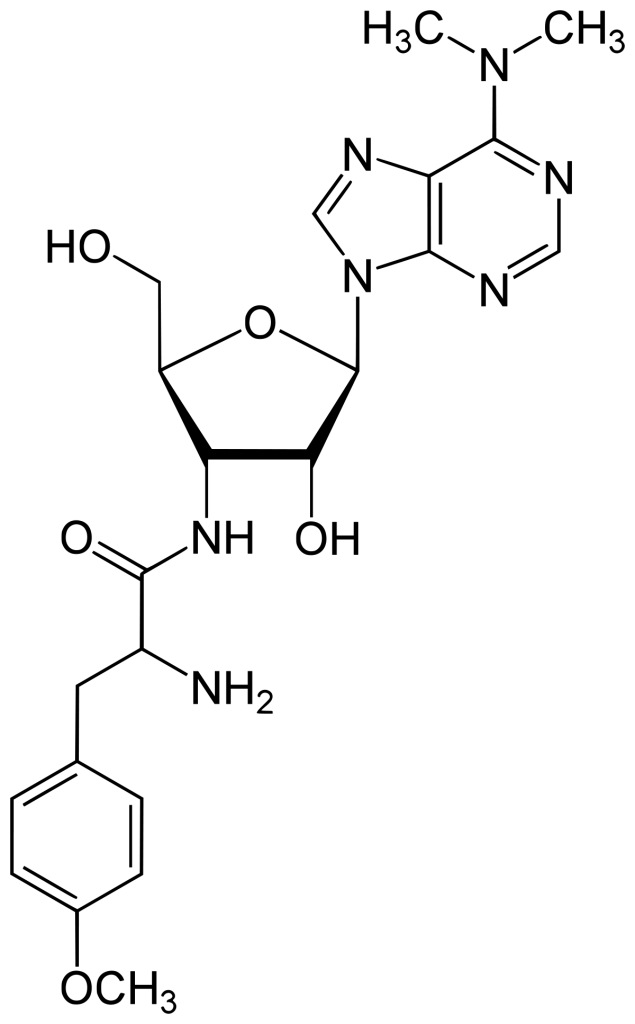
Puromycin
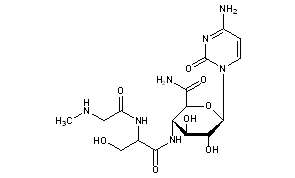
Gougerotin
Blasticidin S
FAQ
Amicetin is an antibacterial and antiviral agent belonging to a group of disaccharide nucleoside antibiotics featuring an \(\alpha-(1 \rightarrow 4)\)-glycosidic bond.
Plant, animal and microorganism tissues are a source of nucleosides that are not present in nucleic acids but are found in the cell in a free state. Most of these compounds are antibiotics. These are known as Nucleoside Antibiotics.
They are used to treat various bacterial and viral diseases and also malignant tumors.
